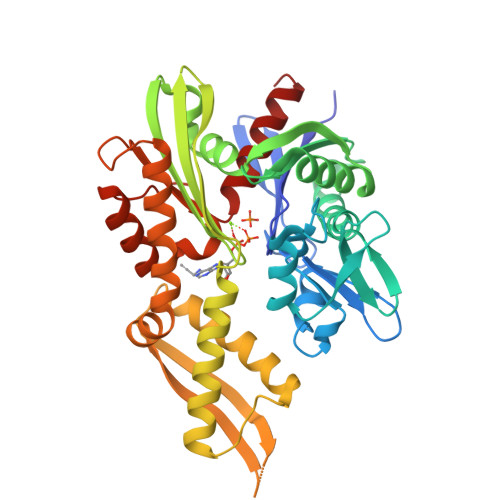
2- and N6-functionalized adenosine-5'-diphosphate analogs for the inhibition of mortalin.
Moseng, M.A., Nix, J.C., Page, R.C.(2019) FEBS Lett 593: 2030-2039
- PubMed: 31177526
- DOI: https://doi.org/10.1002/1873-3468.13475
- Primary Citation of Related Structures:
6P2U - PubMed Abstract:
Our early efforts to find a covalent inhibitor of mortalin, a member of the 70 kD heat shock protein (Hsp70) family, led us to solve the structure of the mortalin nucleotide-binding domain (NBD) in complex with N6-propargyladenosine-5'-diphosphate. The acquired structure emphasizes the ability of the nucleotide-binding pocket to accommodate modified ADP compounds. A library of ADP analogs modified at either the 2- or N6-positions of adenosine was screened against the mortalin-NBD. Competitive inhibition and binding assays of the analogs demonstrate that modifications at the 2- or N6-positions have potential to bind and inhibit mortalin uniquely compared to other Hsp70 homologs, and that modifications at the 2-position confer the greatest selectivity in binding and inhibition of the mortalin-NBD.
Organizational Affiliation:
Department of Chemistry and Biochemistry, Miami University, Oxford, OH, USA.